As a major landmark in drug development for metabolic dysfunction-associated steatohepatitis (MASH; previously known as NASH, nonalcoholic steatohepatitis), the Food and Drug Administration (FDA) recently approved Resmetirom (Rezdiffra®, Madrigal Pharmaceuticals) for management of the disease. Madrigal’s drug received accelerated approval by the FDA, as it fulfils an unmet medical need for a serious health condition with no current effective therapy. FDA’s approval illustrates that Madrigal Pharmaceuticals, a small biotech company, succeeded in a notoriously difficult disease area where several pharma and biotech companies have previously failed due to lack of efficacy or reporting of major adverse effects.
What is MASH?
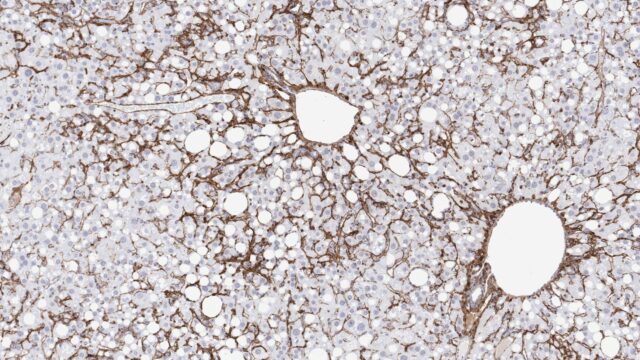
MASH is a common, yet serious form of metabolic dysfunction-associated steatotic liver disease (MASLD; previously known as non-alcoholic fatty liver disease, NAFLD). MASH is diagnosed by liver-biopsy confirmed excess hepatic fat accumulation, lobular inflammation and hepatocyte injury (ballooning). The condition is often co-morbid with type 2 diabetes and obesity which are major risk factors for development and progression of MASLD/MASH. Given the global epidemics of these two metabolic conditions, MASH is affecting millions of people worldwide and has become the leading cause of liver-related mortality. The risk of severe adverse liver outcomes, including hepatocellular carcinoma (HCC), cirrhosis and end-stage liver disease, increase drastically once MASH progresses to advanced liver fibrosis. It is therefore anticipated that MASH will become the leading indication for liver transplantation within the next few years.
What is Resmetirom and what is it used for?
Resmetirom is the first drug to have met both regulatory primary endpoints of resolving MASH and improving fibrosis in a phase 3 clinical trial. Resmetirom, a liver-targeted THR-β receptor agonist, is approved for the treatment of patients with MASH and moderate-to-advanced liver fibrosis (stage F2-F3) in conjunction with diet and exercise. Resmetirom will be administered orally once daily, and Madrigal Pharmaceuticals expects the drug to become available to patients in April 2024.
The current FDA approval is based on an analysis of approximately 966 patients with biopsy-confirmed MASH and liver fibrosis enrolled in the pivotal MAESTRO-NASH clinical phase-3 trial. Resmetirom was administered once daily (80mg or 100mg) and promoted resolution of MASH with no worsening of fibrosis in 25.9% and 29.9% of the patients, respectively, versus 9.7% in the placebo group. Fibrosis improvement by at least 1 stage with no worsening of MASH was achieved in 24.2% and 25.9% of patients in the two treatment arms, as compared 14.2% in the placebo group.
What will happen now?
While being highly encouraging in preclinical and clinical drug discovery, the placebo-adjusted response rate and effect size on liver histological outcomes of Resmetirom was relatively moderate in the MAESTRO-NASH clinical trial. Drug development for MASH is a highly competitive area and there will undoubtedly be a continuous need for drugs with improved efficacy profile, bolstering hopes for highly diverse monotherapies and drug combination concepts in the future management of MASH.
In support of this notion, the pipeline of drugs entering late-stage clinical development for MASH is growing and includes compounds with highly different molecular targets and mechanisms. Most advanced drugs in clinical trials for MASH include incretin mimetics (Semaglutide, Novo Nordisk; Tirzepatide, Eli Lilly; Survodutide, Boehringer-Ingelheim/Zealand Pharma); FGF-21 analogues (Efruxifermin, Akero; Pegozafermin, 89bio/Teva), pan-PPAR agonist (Lanifibranor, Inventiva); HSD17B13 siRNA (Rapirosiran, Regeneron/Alnylam) and PNPLA3 anti-sense ASO (AZD-2693, AstraZeneca).
Implications for preclinical drug discovery
This first FDA drug approval for the liver disease has also implications in preclinical drug discovery, as preclinical studies have until now been characterizing drug candidates in the absence of MASH-targeted drugs approved for clinical use. This has made it challenging to preclinical benchmark drug candidates with therapeutic potential in MASH. Hence, Resmetirom represents an important reference compound for the continued assessment of the translatability and predictive (pharmacological) validity of current animal models of MASH.
The GAN DIO-MASH mouse ranks #1 for clinical translatability
The Gubra-Amylin NASH (GAN) diet-induced obese (DIO) mouse is a golden standard industry-leading model of biopsy-confirmed MASH with progressive fibrosis. This model has been comprehensively validated for human translatability by Gubra and several other research laboratories. The GAN diet is rich in saturated fat, fructose and cholesterol, and consistently induces manifest MASH and fibrosis in C57BL6/J mice after approximately 28 weeks of feeding. The GAN DIO-MASH mouse model recapitulates metabolic, biochemical, histological and molecular hallmarks of MASH. In addition, the GAN DIO-MASH mouse shows progressive HCC development following extended GAN diet feeding. Drugs in late-stage clinical development for MASH have been reported effective in ameliorating MASH, fibrosis and HCC in the GAN DIO-MASH mouse.
The LITMUS consortium has recently ranked the GAN DIO-MASH mouse no. 1 for Human Proximity Score, which incorporates the metabolic phenotype, liver histology and transcriptome signatures benchmarked against human MASH. The LITMUS investigators are internationally recognized experts with substantial expertise within preclinical/clinical research and drug development for MASH.
Characterization of resmetirom in the GAN DIO-MASH mouse
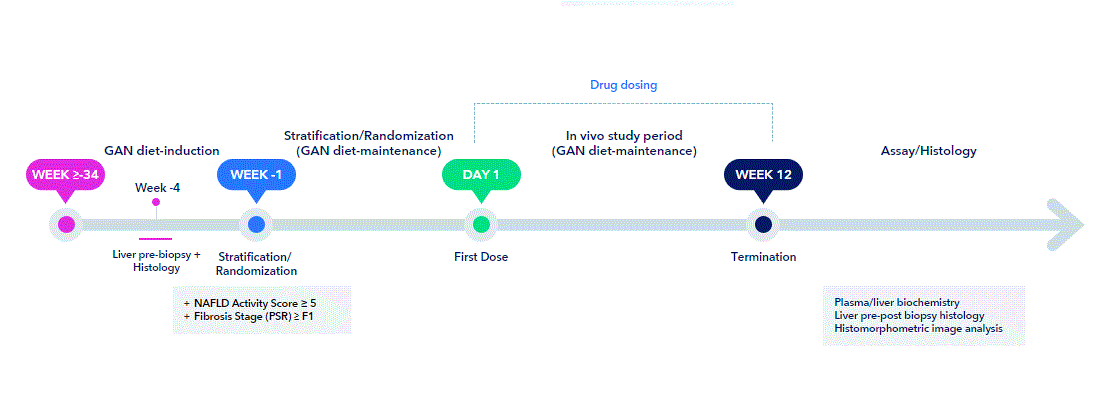
In addition to robustness of the disease phenotype, reproducibility of treatment outcomes is critical for selection of the appropriate preclinical model of MASH. We therefore conducted a comprehensive series of studies in GAN DIO-MASH mice to investigate changes in histological endpoints following 12 weeks of monotherapy with drugs in late-stage clinical development for MASH, including Resmetirom, Semaglutide, Lanifibranor, Elafibranor, Obeticholic acid and Firsocostat. To enable stratification of baseline disease, only mice with biopsy-confirmed MASH (NAS≥5) and fibrosis (stage ≥F1) were included.
1. Clinical translatability of the GAN diet-induced obese and biopsy-confirmed mouse model of MASH
A direct comparison with corresponding clinical trial data was accomplished by evaluating liver histological outcomes with reference to FDA/EMA-accepted histological endpoints. We have summarized results from the GAN DIO-MASH mouse intervention studies here.
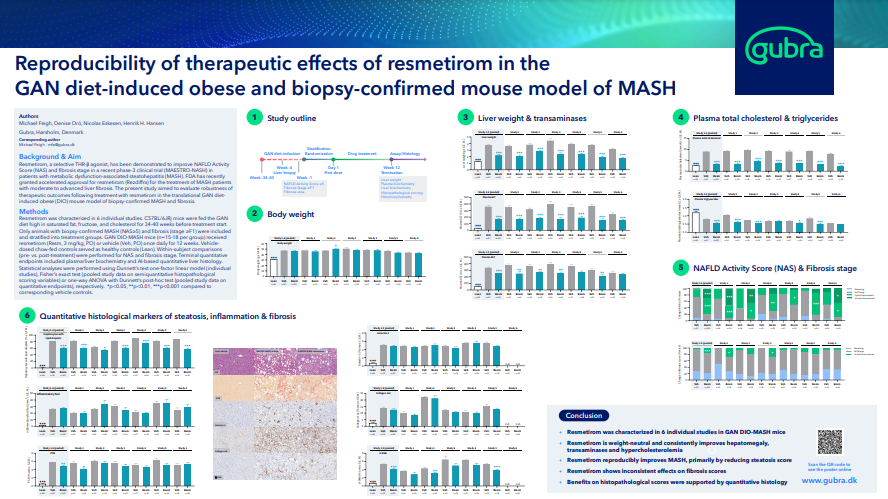
2. Reproducibility of therapeutic effects of resmetirom in the GAN diet-induced obese and biopsy-confirmed mouse model of MASH
In addition, we performed a further detailed analysis of the reproducibility and preclinical effects of Resmetirom on biomarkers, histopathological scores and quantitative histological endpoints in GAN DIO-MASH mice. These data can be downloaded here.
Based on comparative analysis of 6 individual GAN DIO-MASH mouse studies with identical design, we conclude that Resmetirom:
- is weight-neutral
- consistently improves hepatomegaly, transaminases and hypercholesterolemia
- response rate on clinical primary histological endpoints is comparable with the MAESTRO-NASH trial
- reproducibly improves MASH, primarily by reducing steatosis
- show less consistent effects on histological markers of fibrosis
Hence, therapeutic outcomes of Resmetirom treatment are reproducible in GAN DIO-NASH mice and overall consistent with the MAESTRO-NASH clinical trial, further supporting clinical translatability of the model.
Get expert scientific guidance in preclinical drug development for MASH
It is crucial to choose the optimal MASH model and obtain conclusive data to progress preclinical drug candidates. We have 10+ years of experience in conducting preclinical MASH studies with numerous pharmaceutical and biotech companies. Gubra serves 15 out of the 20 largest pharmaceutical companies in the world.
Gubra is industry-leading in translational models of MASH and MASH-driven HCC. We offer tailored design and rapid initiation and turnaround of your study. Our automated AI-based histology pipeline enables effective, accurate and objective analysis of drug effects on clinically relevant endpoints.
If you like to explore our portfolio of MASH models and discover why other companies have chosen us as their trusted preclinical research partner, do not hesitate to contact us.