Cro services in
Liver (NASH/MASH)
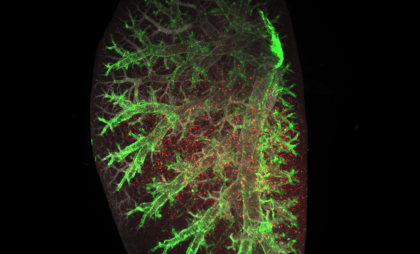
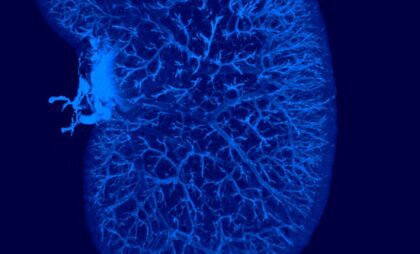
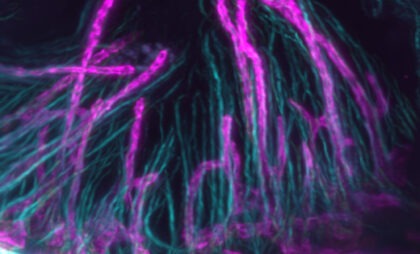
Gubra is industry-leading in translational models of non-alcoholic steatohepatitis (NASH*) and NASH-driven hepatocellular carcinoma (NASH-HCC). Our automated AI-based histology pipeline enables effective, accurate and objective analysis of drug effects on pivotal clinical endpoints.
*Metabolic dysfunction-associated steatohepatitis (MASH) is now the replacement term for NASH.
Why Gubra?
- Expert scientific guidance in NASH/MASH
- Clinically translatable models
- GAN DIO-NASH (GAN DIO-MASH) mouse ranked #1 by LITMUS consortium
- Biopsy-enabled stratification of baseline disease
- Profile compounds using clinical trial endpoints
- Tailored study design and rapid initiation


Consult with Michael Feigh
Vice President, Scientific Sales
Choose the right animal model for your NASH/MASH-targeted drug candidate.
What is MASH?
Metabolic Dysfunction-associated Steatohepatitis (MASH)
The terms non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) will now be replaced by MASLD (metabolic dysfunction-associated steatotic liver disease) and MASH (metabolic dysfunction-associated steatohepatitis).
Industry-leading models for translatability into the clinic
Choosing the right translational NASH/MASH model is crucial for efficacy evaluation and bridging to clinical development. Our GAN DIO-NASH (GAN DIO-MASH) mouse model has been ranked #1 by the LITMUS consortium for its human proximity score and clinical translatability. Our model is already induced and ready to be used for your study immediately. All animals are biopsy-confirmed for stratification of baseline disease.
Main Offerings
Full metabolic
assessment
Biochemical assessment
liver & plasma
NAS & fibrosis scoring
(pre-post)
Quantitative histology
Steatohepatitis & fibrosis
Hepatic transcriptomic
profile
GAN DIO-NASH (GAN DIO-MASH)
mice readily available
High clinical translatability
Models of NASH/MASH & NASH/MASH-driven HCC
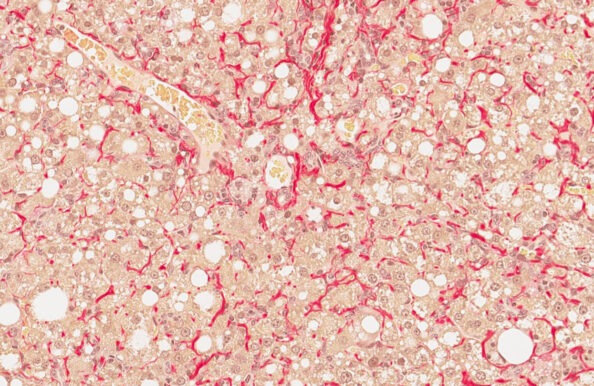
GAN DIO-NASH mouse
(GAN DIO-MASH mouse)
- Ranked #1 for human translatability by the LITMUS consortium
- Diet-induced obesity with metabolic disease
- Fibrosing NASH/MASH ≥28 weeks of GAN dieting
- Biopsy-confirmed NASH/MASH and fibrosis
- Clinical histopathological scoring (pre-post)
- Validated with clinical late-stage drugs
- Therapeutic intervention
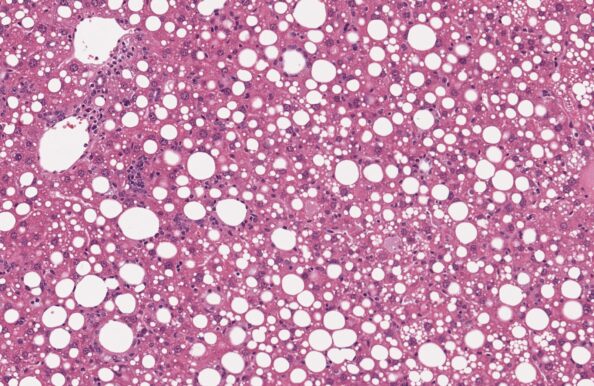
GAN DIO-NASH-HCC mouse
(GAN DIO-MASH-HCC mouse)
- Extended GAN DIO-NASH (GAN DIO-MASH) model
- HCC development ≥ 48 weeks of GAN diet feeding
- Biopsy-confirmed NASH/MASH and fibrosis
- Clinical histopathological scoring (pre-post)
- Macroscopic HCC evaluation
- Validated with clinical late-stage drugs
- Therapeutic/Prophylactic intervention
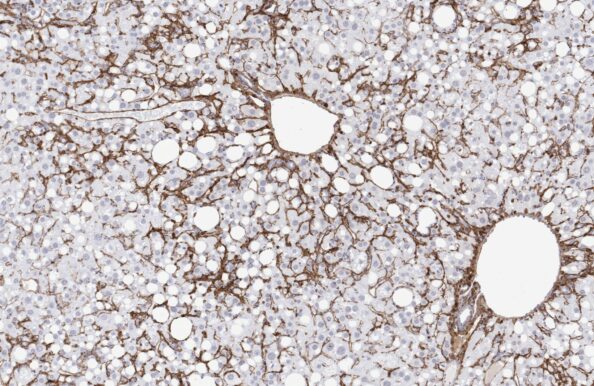
GAN ob/ob-NASH mouse
(GAN ob/ob-MASH mouse)
- Top-ranked for human translatability by the LITMUS consortium
- Accelerated NASH / MASH with progressive advanced fibrosis
- GAN diet feeding for ≥12 weeks
- Genetic obesity with severe metabolic disease
- Clinical histopathological scoring
- Validated with clinical late-stage drugs
- Therapeutic/Prophylactic intervention
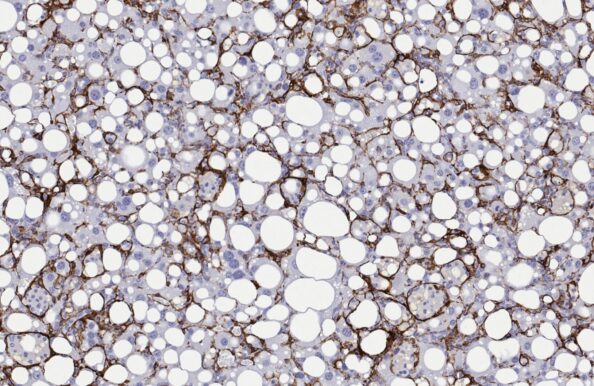
CDAA-HFD mouse
- Recommended by the LITMUS consortium
- Accelerated NASH/MASH with progressive advanced fibrosis
- CDAA-HFD administration for ≥12 weeks
- Non-obese phenotype
- Clinical histopathological scoring (post)
- Macroscopic tumor evaluation
- Validated with clinical late-stage drugs
- Therapeutic/Prophylactic intervention
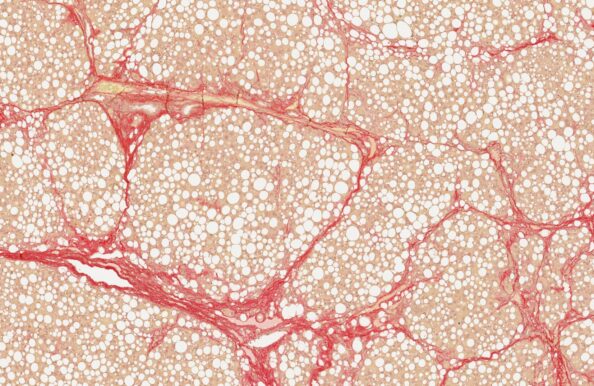
CDAA-HFD rat
- Accelerated NASH/MASH with progression to cirrhosis
- CDAA-HFD administration for up to 16 weeks
- Non-obese phenotype
- Biopsy-confirmed NASH/MASH and fibrosis
- Clinical histopathological scoring (pre-post)
- Macroscopic tumor evaluation
- Validated with clinical late-stage drugs
- Therapeutic/Prophylactic intervention
Related pages
For further information
Contact us
Gubra
Hørsholm Kongevej 11B
2970 Hørsholm
Denmark
+45 3152 2650