Step-by-step guide
Study Execution
Gubra offers comprehensive preclinical CRO solutions from designing studies to data reporting and medical writing. Your designated Gubra Study Director will keep you updated on the study progress on a regular basis and help you interpret the data report, ensuring transparent communication throughout the process.
What is included:
- Designated Study Director with expertise
- Access to our centralized data platform GubraView
- Regular updates on study progress and data
- Data Report and Executive Summary
- Raw data package
- Medical writing service (optional)

Consult with Dagne Barbuskaite
Research Scientist
Experience the difference of having a dedicated study team that prioritizes your research needs.
complete preclinical research solutions
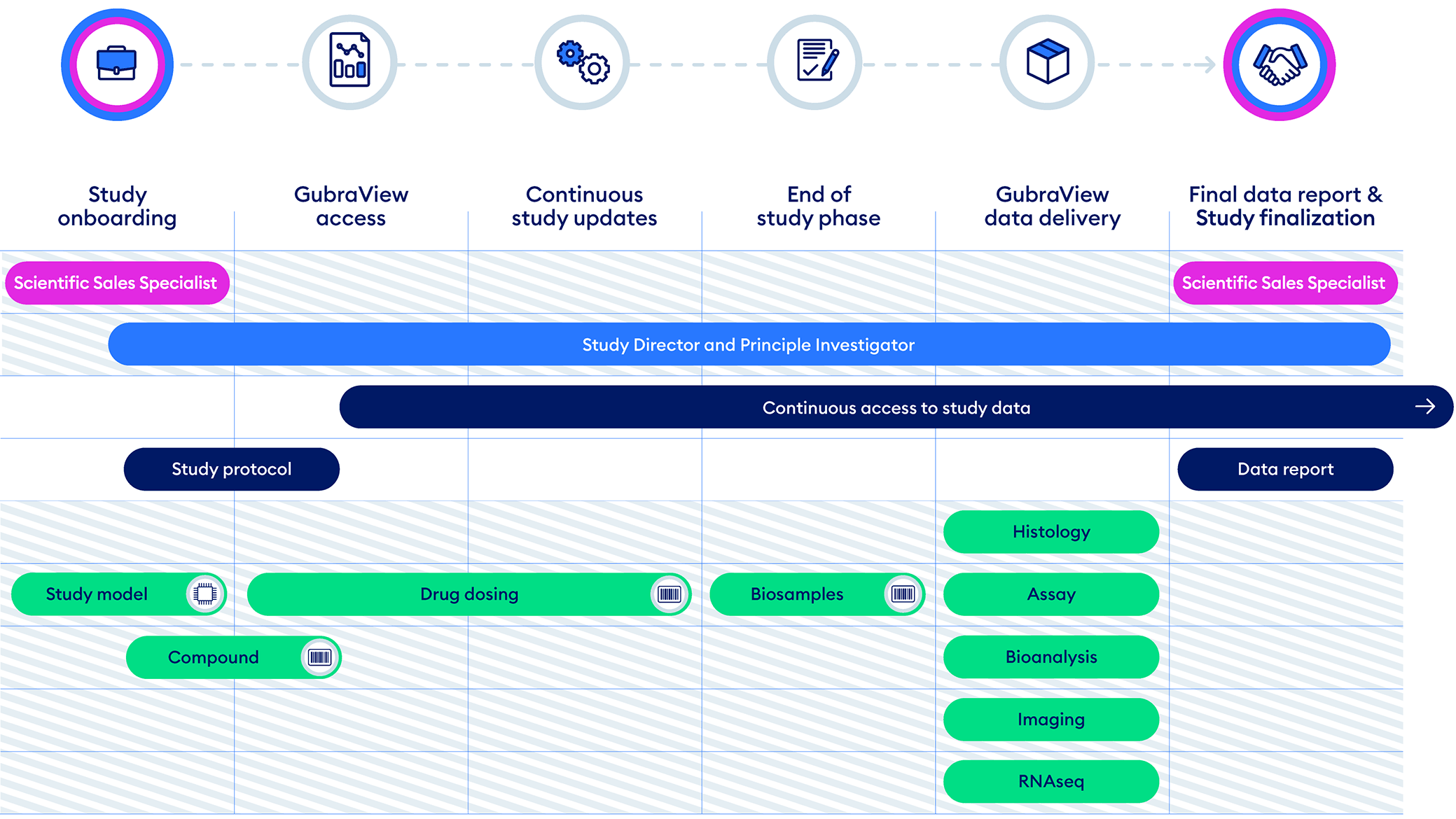
Study Execution Overview
Explore how Gubra conducts a preclinical study as a trusted CRO partner. To view more details, hover your mouse cursor over each step.

Gubra’s research team consists of 200+ skilled scientists and technicians with expertise in metabolic and fibrotic diseases. Every study at Gubra is handled by a dedicated Study Team throughout the whole process, facilitating continuous and transparent communications with the sponsor company.

“
We are dedicated to delivering conclusive results to help you make the right decision.
As an experienced preclinical CRO, we understand the importance of conclusive study data in progressing the drug pipeline. From designing the study to interpreting its results, we will assist you with know-how from 15+ years of successful experience.
Related pages
For further information
Contact us
Gubra
Hørsholm Kongevej 11B
2970 Hørsholm
Denmark
+45 3152 2650



